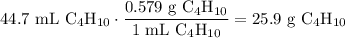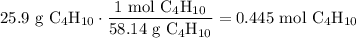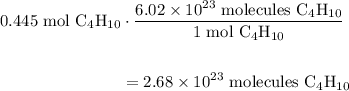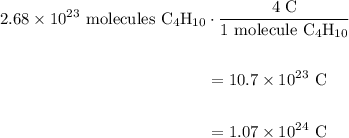Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1
You know the right answer?
A beaker contains 44.7 mL of butane ( C4H10, density is 0.579 g/mL).
Determine how many C atoms th...
Questions

Social Studies, 04.05.2020 23:09

Mathematics, 04.05.2020 23:09

Mathematics, 04.05.2020 23:09

Spanish, 04.05.2020 23:09


English, 04.05.2020 23:09

Mathematics, 04.05.2020 23:09



Mathematics, 04.05.2020 23:09

Mathematics, 04.05.2020 23:09

Mathematics, 04.05.2020 23:09



Mathematics, 04.05.2020 23:09

Mathematics, 04.05.2020 23:09




Mathematics, 04.05.2020 23:09