
Chemistry, 27.09.2021 14:00 xscape0mari
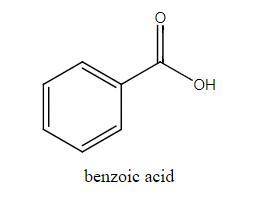
The molar mass of benzoic acid (C6H5COOH) determined by measuring the freezing‐point depression in benzene is twice what we would expect for the molecular formula, C7H6O2. Explain this apparent anomaly. Thank you! :)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1


Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

You know the right answer?
The molar mass of benzoic acid (C6H5COOH) determined by measuring the freezing‐point depression in b...
Questions








History, 11.01.2020 05:31


Mathematics, 11.01.2020 05:31

Mathematics, 11.01.2020 05:31

Mathematics, 11.01.2020 05:31


Mathematics, 11.01.2020 05:31




Mathematics, 11.01.2020 05:31




