
Chemistry, 29.09.2021 20:30 Katiecool290
STEM 11
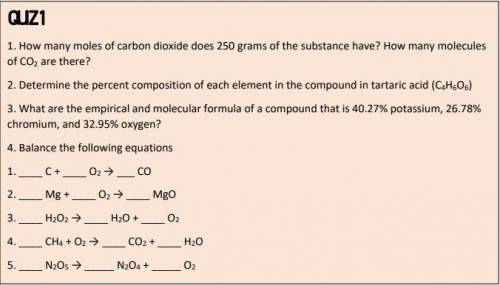
1.How many moles of carbon dioxide does 250 grams of the substance have? How many molecules of CO2 are there?
2. Determine the percent composition of each element in the compound in tartaric acid (C4H6O6)
3. What are the empirical and molecular formula of a compound that is 40.27% potassium, 26.78%
chromium, and 32.95% oxygen?
4. Balance the following equations
1. _ C + _ O2 → ___ CO
2. _ Mg + _ O2 → _ MgO
3. _ H2O2 → _ H2O + _ O2
4. _ CH4 + O2 → _ CO2 + _ H2O
5. _ N2O5 → _ N2O4 + _ O2

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
STEM 11
1.How many moles of carbon dioxide does 250 grams of the substance have? How many molecule...
Questions

Chemistry, 19.05.2020 13:08

Mathematics, 19.05.2020 13:08




Arts, 19.05.2020 13:08

Mathematics, 19.05.2020 13:08



Physics, 19.05.2020 13:08


Mathematics, 19.05.2020 13:08

Mathematics, 19.05.2020 13:08

English, 19.05.2020 13:08


Biology, 19.05.2020 13:08






