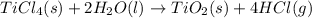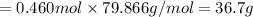Chemistry, 29.09.2021 21:10 kobiemajak
When TiCl4 (s) reacts with H20 (l), the products TiO2 (s) and HCl (g) are formed. If there is 87.3 g of
titanium (IV) chloride present, with water in excess, how much solid titanium (IV) oxide (in grams)
could theoretically be produced?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3


Chemistry, 23.06.2019 10:00
Lord kelvin described the concept of absolute zero temperature and the laws relating to the change of thermal energy during chemical reactions what type of chemist would he be considered today
Answers: 1
You know the right answer?
When TiCl4 (s) reacts with H20 (l), the products TiO2 (s) and HCl (g) are formed. If there is 87.3 g...
Questions








History, 21.07.2019 07:30

History, 21.07.2019 07:30

Mathematics, 21.07.2019 07:30

Physics, 21.07.2019 07:30

Social Studies, 21.07.2019 07:30

History, 21.07.2019 07:30




Mathematics, 21.07.2019 07:30

Health, 21.07.2019 07:30

Mathematics, 21.07.2019 07:30