
Chemistry, 08.10.2021 01:50 alwayspouty624
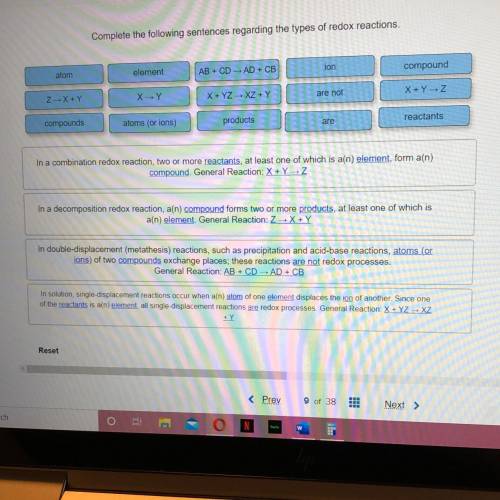
N a combination redox reaction, two or more REACTANTS, at least one of which is an ELEMENT, form a COMPOUND. General reaction: X+Y->Z
In a decomposition redox reaction, a COMPOUND forms two or more PRODUCTS, at least one of which is an ELEMENT. General reaction: Z->X+Y
In double-displacement (metathesis) reactions, such as precipitation and acid-base reactions, ATOMS (OR IONS) of two COMPOUNDS exchange places; these reactions ARE NOT redox processes. General reaction: AB+CD->AD+CB
In solution, single-displacement reactions occur when an ATOM of one ELEMENT displaces the ION of another. Since one of the REACTANTS is an ELEMENT, all single-displacement reactions ARE redox processes. General reaction: X+YZ->XZ+Y
Verified by assignments "Check your work mode."

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2
You know the right answer?
N a combination redox reaction, two or more REACTANTS, at least one of which is an ELEMENT, form a C...
Questions

Mathematics, 28.01.2021 15:00



Mathematics, 28.01.2021 15:00

Mathematics, 28.01.2021 15:00

Mathematics, 28.01.2021 15:00

Mathematics, 28.01.2021 15:00

Arts, 28.01.2021 15:00

Law, 28.01.2021 15:00

English, 28.01.2021 15:00

Mathematics, 28.01.2021 15:10


Mathematics, 28.01.2021 15:10

Mathematics, 28.01.2021 15:10


Mathematics, 28.01.2021 15:10


Computers and Technology, 28.01.2021 15:10

Mathematics, 28.01.2021 15:10

Social Studies, 28.01.2021 15:10



