
Chemistry, 10.10.2021 03:20 olejlund8073
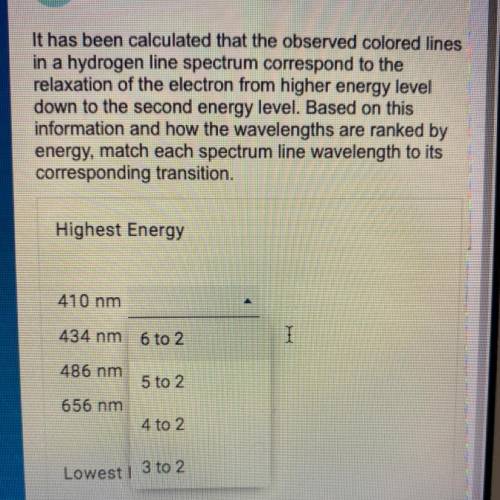
It has been calculated that the observed colored lines in a hydrogen line spectrum correspond to the relaxation of the electron from higher energy level down to the second energy level. Based on this information and how the wavelengths are ranked by energy, match each spectrum line wavelength to its corresponding transition.

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
It has been calculated that the observed colored lines in a hydrogen line spectrum correspond to the...
Questions



Biology, 01.01.2020 01:31



History, 01.01.2020 01:31


History, 01.01.2020 01:31

Arts, 01.01.2020 01:31

Mathematics, 01.01.2020 01:31







Mathematics, 01.01.2020 01:31


Mathematics, 01.01.2020 01:31



