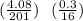Chemistry, 10.10.2021 21:40 skyemichellec
A compound of mercury and oxygen is heated in order to decompose the compound. A 4.08 grams sample of mercury oxide upon heating gives off oxygen gas. After heating the mass of mercury metal left behind was 3.78 grams. Calculate the empirical formula of the compound
My and: 1 O2 to 2 Hg ratio but I don't know if it's correct and if so if I should write it as
2HgO > 2Hg + O2 or just 2Hg + O2
Please someone who understands help.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
A compound of mercury and oxygen is heated in order to decompose the compound. A 4.08 grams sample o...
Questions


Mathematics, 12.08.2020 09:01

Mathematics, 12.08.2020 09:01


English, 12.08.2020 09:01





Social Studies, 12.08.2020 09:01









Mathematics, 12.08.2020 09:01