
Chemistry, 11.10.2021 14:00 babygirl2984
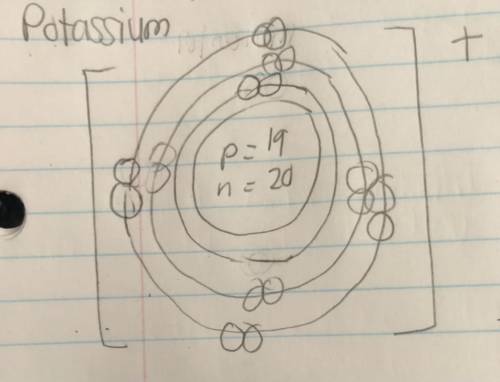
QUICK QUESTION: On the Bohr model, how come potassium has 19 electrons in its valence shell if potassium has a K+? Isn’t it suppose to have 18 electrons? I thought that if an ion has a positive charge, the atom has lost electrons. Pls help me I WILL GIVE BRAINLIEST TO THE BEST ANSWER

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3
You know the right answer?
QUICK QUESTION: On the Bohr model, how come potassium has 19 electrons in its valence shell if potas...
Questions

Physics, 28.07.2019 23:30

Mathematics, 28.07.2019 23:30


Social Studies, 28.07.2019 23:30


Physics, 28.07.2019 23:30

English, 28.07.2019 23:30

Mathematics, 28.07.2019 23:30



English, 28.07.2019 23:30



Advanced Placement (AP), 28.07.2019 23:30

Physics, 28.07.2019 23:30


History, 28.07.2019 23:30

Mathematics, 28.07.2019 23:30




