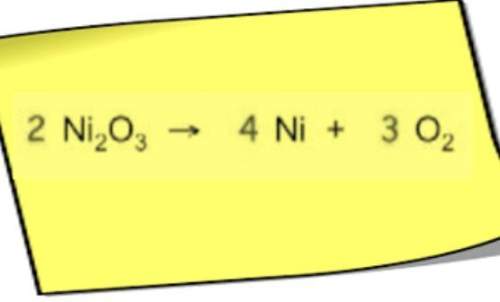
Chemistry, 11.10.2021 14:30 michaelortega5808
An analysis of a substance determined that the substance contains carbon, hydrogen
and oxygen. What is the empirical formula given that the following quantities were
present: 24.19 g C, 4.06 g Hand 26.75 g 0.
You won't be able to enter subscripts, so just enter the number where it goes.
For example, S203.
Your formula should have the following order of atoms: CHO

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
An analysis of a substance determined that the substance contains carbon, hydrogen
and oxygen. Wha...
Questions

Physics, 25.01.2022 02:20

Mathematics, 25.01.2022 02:20

Mathematics, 25.01.2022 02:20



Mathematics, 25.01.2022 02:20

Biology, 25.01.2022 02:20

Mathematics, 25.01.2022 02:20

Mathematics, 25.01.2022 02:20

History, 25.01.2022 02:20


Chemistry, 25.01.2022 02:20


Mathematics, 25.01.2022 02:20


Biology, 25.01.2022 02:20



Chemistry, 25.01.2022 02:20