
ATOMS-
1. an atom is the smallest part of a pure substance which retains the _ of that pure substance
COMPOUNDS-
2. atoms of each element are essentially the _
LAW OF DEFINITE PROPORTIONS-
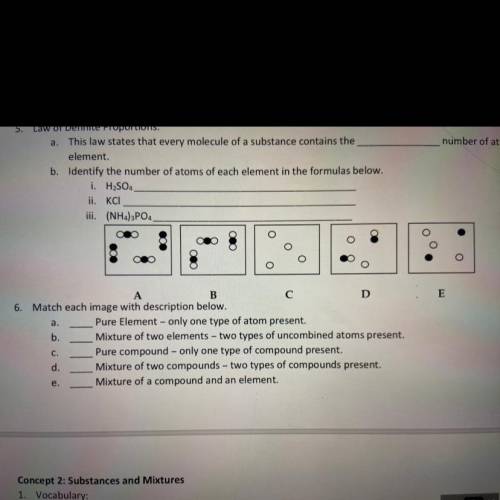
3. this law states that every molecule of a substance contains the _ number of atoms of each element
SOLUTIONS-
4. Solutions are special types of mixtures which are _ so they often can be confused as pure substances
STATES OF MATTER-
5. how would you describe the change in arrangement of particles as heat energy and temperature increases?
6. why does the temperature remain constant during a phase change even though the substance is absorbing heat energy?
7. (number six only)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
ATOMS-
1. an atom is the smallest part of a pure substance which retains the _ of that pure substa...
Questions

Biology, 26.04.2020 00:47



Social Studies, 26.04.2020 00:47

Mathematics, 26.04.2020 00:50



History, 26.04.2020 00:50


Mathematics, 26.04.2020 00:50



Health, 26.04.2020 00:50


Physics, 26.04.2020 00:50







