
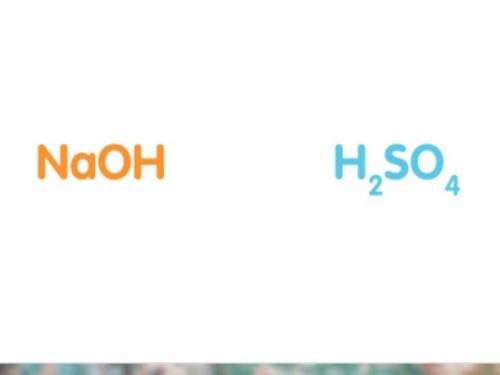
50cm3 of sodium hydroxide solution was titrated against a solution of sulfuric acid. The concentration of the sodium hydroxide solution was 20g/dm3. Work out the concentration of the acid in moles per litre if it took 25cm3 of acid to completely neutralise the alkali. (Hint: It may help to write out a balanced symbol equation for the reaction. The chemical formulas of the acid and alkali are shown below.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
50cm3 of sodium hydroxide solution was titrated against a solution of sulfuric acid. The concentrati...
Questions


English, 23.07.2019 15:00

Health, 23.07.2019 15:00


History, 23.07.2019 15:00


Chemistry, 23.07.2019 15:00


History, 23.07.2019 15:00

Health, 23.07.2019 15:00

Mathematics, 23.07.2019 15:00

English, 23.07.2019 15:00

Spanish, 23.07.2019 15:00


Mathematics, 23.07.2019 15:00

Mathematics, 23.07.2019 15:00

Biology, 23.07.2019 15:00


Social Studies, 23.07.2019 15:00

Mathematics, 23.07.2019 15:00



