6
2 points
For the following reaction, what is the theoretical yield in grams of H2O when 05...

Chemistry, 13.10.2021 14:00 noberoger2780
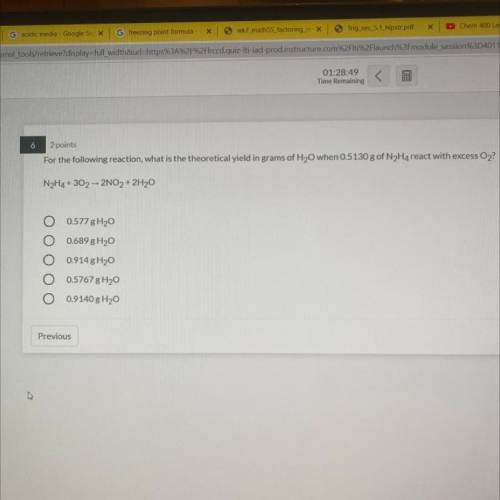
6
2 points
For the following reaction, what is the theoretical yield in grams of H2O when 05130 g of N Ha react with excess O2
NH4+ 30, 2NO, + 2H30
O 0577 8H20
0.689 H2O
ООООО
0914 8H2O
O 0.5767 g 120
O 0.9140 g Hy

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2
You know the right answer?
Questions

Mathematics, 04.04.2020 01:50

Mathematics, 04.04.2020 01:50







Mathematics, 04.04.2020 01:51



English, 04.04.2020 01:51


Mathematics, 04.04.2020 01:51




Arts, 04.04.2020 01:51

Mathematics, 04.04.2020 01:51



