
Chemistry, 14.10.2021 01:00 abelinoperez652
if 1.40 mol mg are mixed with 2.00 mol of HCl, how many moles of excess reactant remain after the reaction is complete?
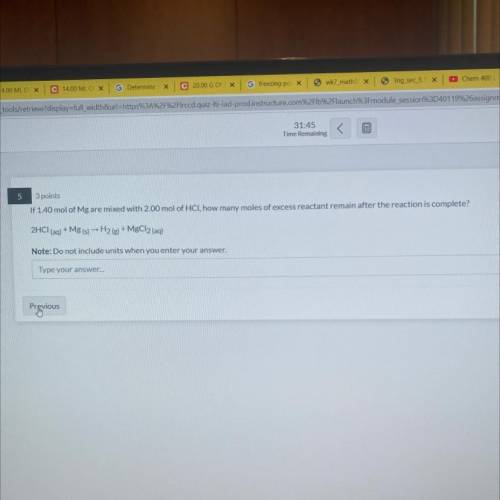

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 21.06.2019 17:30
What is the percentage by mass of silicon (si) in iron aluminum silicate (fe3al2(sio4)3)?
Answers: 2


Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2
You know the right answer?
if 1.40 mol mg are mixed with 2.00 mol of HCl, how many moles of excess reactant remain after the re...
Questions

Social Studies, 22.04.2020 15:28




Biology, 22.04.2020 15:29

Computers and Technology, 22.04.2020 15:29




Health, 22.04.2020 15:29

World Languages, 22.04.2020 15:29



Mathematics, 22.04.2020 15:29

Mathematics, 22.04.2020 15:30

Mathematics, 22.04.2020 15:30

English, 22.04.2020 15:30



Chemistry, 22.04.2020 15:30



