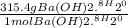WILL GIVE BRAINLIEST AND 20 POINTS! Barium hydroxide, often used to titrate weak organic acids, is obtained as the octahydrate, Ba(OH)2 * 8 H2O. What mass of Ba(OH)2 * 8 H2O would be required to make 500 mL of a solution that is 0.1500 M hydroxide ions? [hint: calculate the molar mass of barium hydroxide octahydrate].

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1

Chemistry, 23.06.2019 04:20
Malia was able to make a paper clip float on the surface of water what will most likely happen to the paper clip if a drop of dishwashing detergent is added near it
Answers: 1

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1
You know the right answer?
WILL GIVE BRAINLIEST AND 20 POINTS!
Barium hydroxide, often used to titrate weak organic acids, is...
Questions

Mathematics, 13.12.2021 04:20

Spanish, 13.12.2021 04:20

Chemistry, 13.12.2021 04:20

Advanced Placement (AP), 13.12.2021 04:20



Advanced Placement (AP), 13.12.2021 04:20

History, 13.12.2021 04:20



Biology, 13.12.2021 04:20


History, 13.12.2021 04:20

Arts, 13.12.2021 04:20

Mathematics, 13.12.2021 04:20

Social Studies, 13.12.2021 04:20

Arts, 13.12.2021 04:20

Mathematics, 13.12.2021 04:20


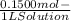 ×
×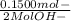 ×
×