O
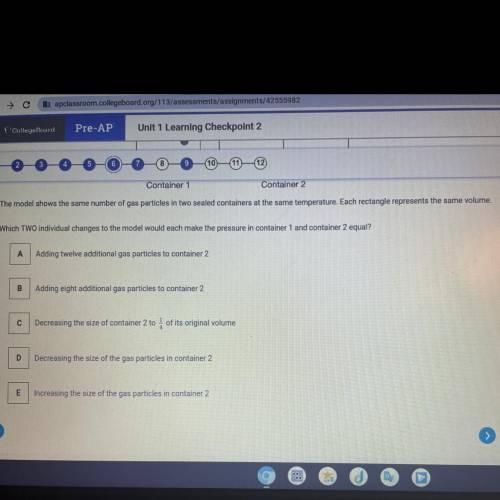
Container 1
Container 2
The model shows the same number of gas particles in two seal...

Chemistry, 20.10.2021 14:00 pleasehelpme666
O
Container 1
Container 2
The model shows the same number of gas particles in two sealed containers at the same temperature. Each rectangle represents the same volume.
Which TWO individual changes to the model would each make the pressure in container 1 and container 2 equal?
A Adding twelve additional gas particles to container 2
B
Adding eight additional gas particles to container 2
с
Decreasing the size of container 2 to 4 of its original volume

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

You know the right answer?
Questions

Physics, 04.11.2020 20:10






Mathematics, 04.11.2020 20:10


Mathematics, 04.11.2020 20:10




History, 04.11.2020 20:10

Mathematics, 04.11.2020 20:10



Advanced Placement (AP), 04.11.2020 20:10





