
Chemistry, 23.10.2021 19:10 spazzinchicago
Aluminum (AI) and silver tarnish (Ag²S) yield pure silver (Ag) in an aluminum sulfide solution. What are the reactants and products in this reaction?
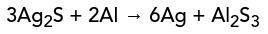

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
Aluminum (AI) and silver tarnish (Ag²S) yield pure silver (Ag) in an aluminum sulfide solution.
Wh...
Questions

English, 29.06.2021 06:50



Mathematics, 29.06.2021 07:00

Health, 29.06.2021 07:00

Mathematics, 29.06.2021 07:00

Advanced Placement (AP), 29.06.2021 07:00

Mathematics, 29.06.2021 07:00

Biology, 29.06.2021 07:00



Mathematics, 29.06.2021 07:00


Mathematics, 29.06.2021 07:00

Mathematics, 29.06.2021 07:00



History, 29.06.2021 07:00





