
Chemistry, 23.10.2021 20:50 raquelrivera03
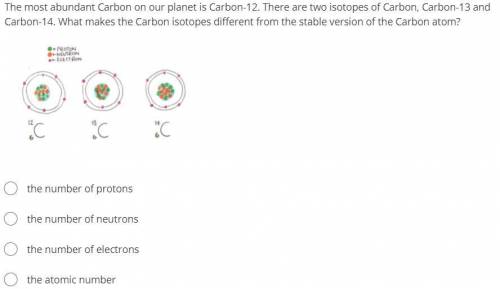
The most abundant Carbon on our planet is Carbon-12. There are two isotopes of Carbon, Carbon-13 and Carbon-14. What makes the Carbon isotopes different from the stable version of the Carbon atom?
the number of protons
the number of neutrons
the number of electrons
the atomic number

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3


Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
The most abundant Carbon on our planet is Carbon-12. There are two isotopes of Carbon, Carbon-13 and...
Questions



English, 23.04.2020 18:06


Mathematics, 23.04.2020 18:06



Geography, 23.04.2020 18:06

Biology, 23.04.2020 18:06



History, 23.04.2020 18:06


Mathematics, 23.04.2020 18:06






Chemistry, 23.04.2020 18:06



