
Chemistry, 24.10.2021 08:20 jennelledenise
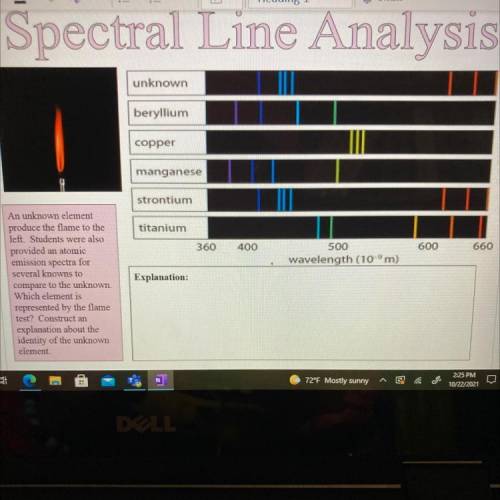
An unknown element
produce the flame to the
left. Students were also
provided an atomic
emission spectra for
several knowns to
compare to the unknown.
Which element is
represented by the flame
test? Construct an
explanation about the
identity of the unknown
element.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2
You know the right answer?
An unknown element
produce the flame to the
left. Students were also
provided an atomi...
left. Students were also
provided an atomi...
Questions

History, 17.10.2019 03:30

Mathematics, 17.10.2019 03:30



English, 17.10.2019 03:30


History, 17.10.2019 03:30

History, 17.10.2019 03:30

Mathematics, 17.10.2019 03:30

Computers and Technology, 17.10.2019 03:30




Mathematics, 17.10.2019 03:30

Chemistry, 17.10.2019 03:30


English, 17.10.2019 03:30


Mathematics, 17.10.2019 03:30

Mathematics, 17.10.2019 03:30



