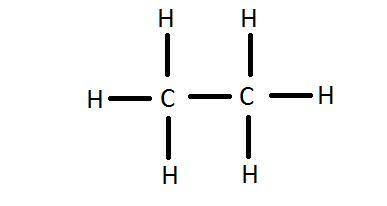
(B) CH3COCH3

Chemistry, 24.10.2021 22:00 sabrinarasull1pe6s61
Which of the following molecules contains only single bonds?
(A)CH3CH2COOH
(B) CH3COCH3
(C) HCN
(D)C2H6
(E) CO2

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Which of the following molecules contains only single bonds?
(A)CH3CH2COOH
(B) CH3COCH3
(B) CH3COCH3
Questions


Mathematics, 25.12.2020 14:00

Mathematics, 25.12.2020 14:00

Chemistry, 25.12.2020 14:00

English, 25.12.2020 14:00

History, 25.12.2020 14:00

Social Studies, 25.12.2020 14:00




Mathematics, 25.12.2020 14:00


Mathematics, 25.12.2020 14:00

Mathematics, 25.12.2020 14:00


Mathematics, 25.12.2020 14:00


Mathematics, 25.12.2020 14:00


Computers and Technology, 25.12.2020 14:00