
Chemistry, 25.10.2021 18:00 angelinaavila06
Determine the molar solubility for Cr(OH)3 Ksp = 6.3x10^-31 .
a) Set up the ICE table Cr(OH)3 (s) <-> Cr^3+ (aq) + 3OH^- (aq)
b) Ksp expression
c) Determine molar solubility
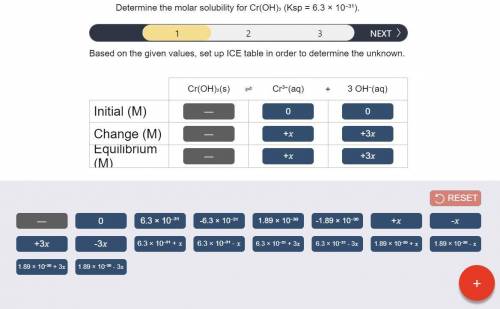
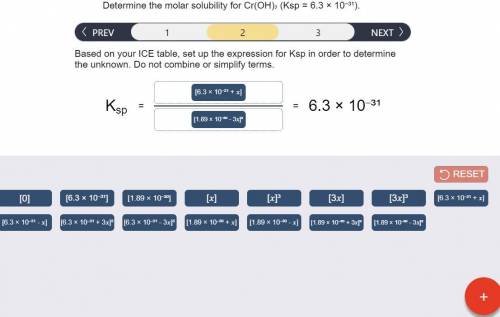
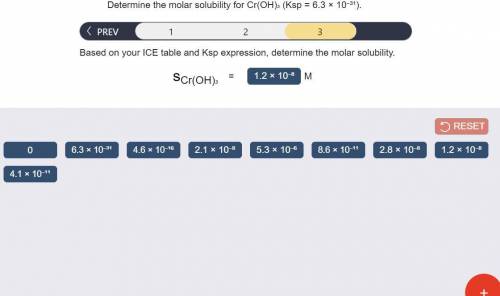

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
Determine the molar solubility for Cr(OH)3 Ksp = 6.3x10^-31 .
a) Set up the ICE table Cr(OH)3 (s)...
Questions


Chemistry, 17.10.2019 21:30

Health, 17.10.2019 21:30

History, 17.10.2019 21:30




Mathematics, 17.10.2019 21:30

Chemistry, 17.10.2019 21:30

Mathematics, 17.10.2019 21:30


Biology, 17.10.2019 21:30




Geography, 17.10.2019 21:30

History, 17.10.2019 21:30



History, 17.10.2019 21:30



