
Chemistry, 28.10.2021 01:00 naiomireyes74p2aybs
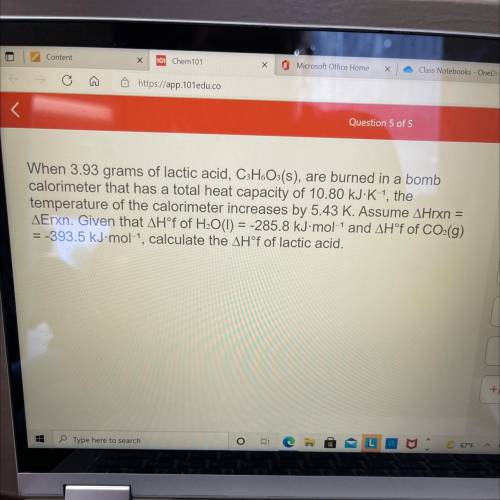
When 3.93 grams of lactic acid, CHoOs(s), are burned in a bomb
calorimeter that has a total heat capacity of 10.80 kJ-K-1, the
temperature of the calorimeter increases by 5.43 K. Assume AHrxn
=
AErxn. Given that AH°f of H¿O(I) = -285.8 kJ-mol-1 and AH°f of CO:(g)
= -393.5 kJ• mol-1, calculate the AH°f of lactic acid.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
When 3.93 grams of lactic acid, CHoOs(s), are burned in a bomb
calorimeter that has a total heat c...
Questions






Biology, 02.07.2019 08:30


Health, 02.07.2019 08:30


English, 02.07.2019 08:30




English, 02.07.2019 08:30


Mathematics, 02.07.2019 08:30



Social Studies, 02.07.2019 08:30



