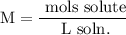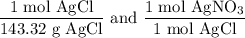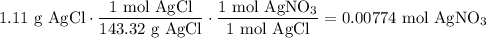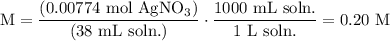Chemistry, 30.10.2021 19:00 jchavez0790
HELPPP PLS IM DESPRATE MAJOR POINTS NEED ASAP
We add excess NaCl solution (58.44 g/mol) to 38 mL of a solution of silver nitrate (AgNO3 169.88 g/mol), to form insoluble solid AgCl. When it has been dried and weighed, the mass of AgCl (143.32 g/mol) is found to be 1.11 grams.
What is the molarity of the original AgNO3 solution? The formula weight of NaNO3 is 85.00 g/mol.
Answer in units of M.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
HELPPP PLS IM DESPRATE MAJOR POINTS NEED ASAP
We add excess NaCl solution (58.44 g/mol) to 38 mL o...
Questions

Mathematics, 10.02.2021 21:00


Mathematics, 10.02.2021 21:00

Mathematics, 10.02.2021 21:00

Mathematics, 10.02.2021 21:00

Mathematics, 10.02.2021 21:00


Mathematics, 10.02.2021 21:00


Mathematics, 10.02.2021 21:00





History, 10.02.2021 21:00

Mathematics, 10.02.2021 21:00

Mathematics, 10.02.2021 21:00


Mathematics, 10.02.2021 21:00

Chemistry, 10.02.2021 21:00