Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2


Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1
You know the right answer?
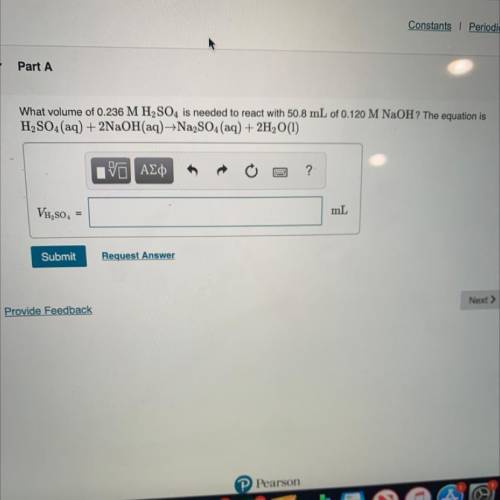
What volume of 0.236 M H2SO4 is needed to react with 50.8 mL of 0.120 M NaOH? The equation is
H2SO...
Questions

Computers and Technology, 16.02.2021 05:40



Mathematics, 16.02.2021 05:40


Advanced Placement (AP), 16.02.2021 05:40


Chemistry, 16.02.2021 05:40



Computers and Technology, 16.02.2021 05:40




Mathematics, 16.02.2021 05:40

Social Studies, 16.02.2021 05:40



Mathematics, 16.02.2021 05:40