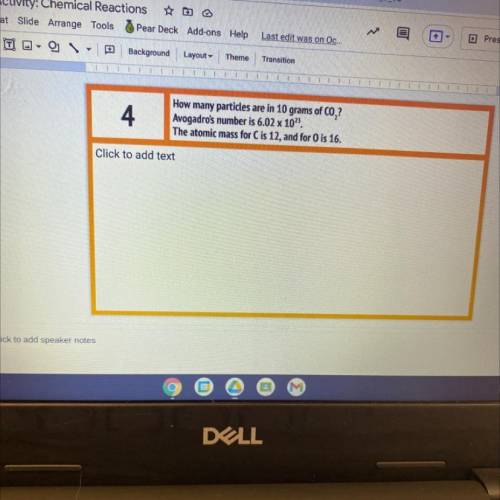
How many particles are in 10 grams of CO,?
Avogadro's number is 6.02 x 10".
The atomic mass...

Chemistry, 06.11.2021 03:50 brialevy2283
How many particles are in 10 grams of CO,?
Avogadro's number is 6.02 x 10".
The atomic mass for C is 12, and for 0 is 16.

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 20:10
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
Questions

Mathematics, 23.09.2019 08:00


Mathematics, 23.09.2019 08:00


Mathematics, 23.09.2019 08:00





Mathematics, 23.09.2019 08:00

Mathematics, 23.09.2019 08:00

Physics, 23.09.2019 08:00






Mathematics, 23.09.2019 08:00

Mathematics, 23.09.2019 08:00




