Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
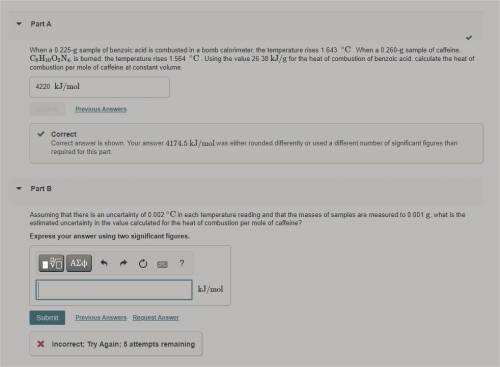
Assuming that there is an uncertainty of 0.002 ∘C in each temperature reading and that the masses of...
Questions


Mathematics, 01.09.2021 01:00

Biology, 01.09.2021 01:00


Mathematics, 01.09.2021 01:00



Biology, 01.09.2021 01:00

Mathematics, 01.09.2021 01:00


Mathematics, 01.09.2021 01:00

Mathematics, 01.09.2021 01:00




Mathematics, 01.09.2021 01:00


Mathematics, 01.09.2021 01:00