
Chemistry, 08.11.2021 14:00 angelolucero146
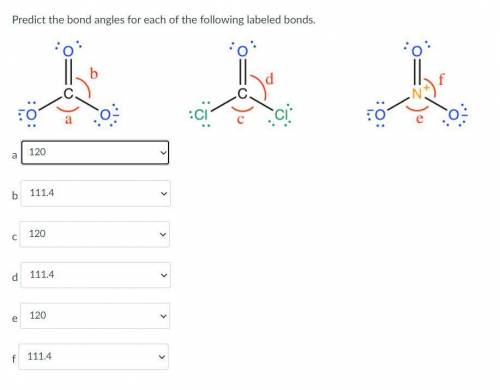
Predict the bond angles for each of the following labeled bonds.
The answer choices are 120, 111.4, and 124.3
See the picture attached.
Note: Here is a link to the answer in case any one else is struggling and needs an explanation:

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
Predict the bond angles for each of the following labeled bonds.
The answer choices are 120, 111.4...
Questions


English, 13.04.2020 12:27


English, 13.04.2020 12:28

History, 13.04.2020 12:28

Chemistry, 13.04.2020 12:28

English, 13.04.2020 12:28

Chemistry, 13.04.2020 12:28

Mathematics, 13.04.2020 12:28



Health, 13.04.2020 12:29

Mathematics, 13.04.2020 12:29


Biology, 13.04.2020 12:47


History, 13.04.2020 12:47

Mathematics, 13.04.2020 12:47

Mathematics, 13.04.2020 12:47




