
Determining Equilibrium
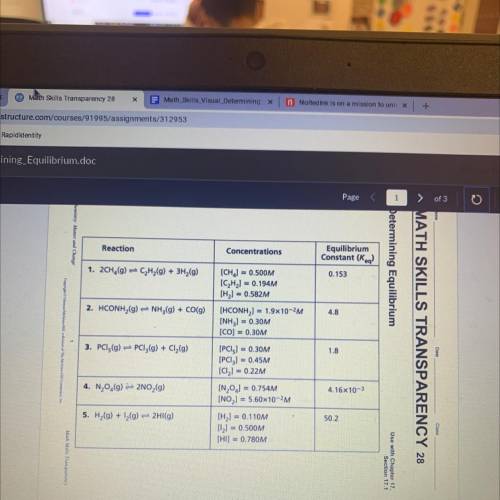
The equilibrium constants for the reactions in the table are correct at a certain
temperature. The concentrations given in the table, however, may or may not be correct
when the system is at equilibrium at that temperature. Use the information in the table to
answer the following questions.
1.On the basis of the K, values given in the table, which reaction mixture contains the largest
amount of product(s) when at equilibrium?
2. Which reaction mixture contains the largest amount of reactants when at
equilibrium? Explain.
3. Which reactions in the table have concentrations that represent the systems at
equilibrium? Explain.
4. For each reaction that is not at equilibrium, change the concentration of only one
of the reactants or products so that the ratio represents the system at equilibrium.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
Determining Equilibrium
The equilibrium constants for the reactions in the table are correct at a...
Questions

English, 28.07.2019 09:30







Computers and Technology, 28.07.2019 09:30

Mathematics, 28.07.2019 09:30



English, 28.07.2019 09:30


English, 28.07.2019 09:30


Mathematics, 28.07.2019 09:30







