
Chemistry, 10.11.2021 20:10 theatergeek005
Topics: Fundamentals of electrochemistry; Electrochemical cells
Use general chemistry rules for significant figures.
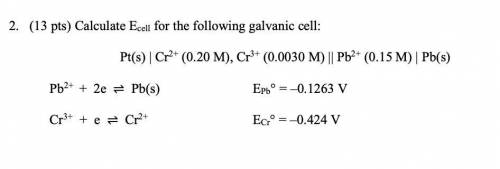
2. (13 pts) Calculate Ecell for the following galvanic cell:
Pt(s) | Cr2+ (0.20 M), Cr3+ (0.0030 M) || Pb2+ (0.15 M) | Pb(s)
Pb2+ + 2e ⇌ Pb(s) EPb° = –0.1263 V
Cr3+ + e ⇌ Cr2+ ECr° = –0.424 V

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

You know the right answer?
Topics: Fundamentals of electrochemistry; Electrochemical cells
Use general chemistry rules for si...
Questions

Business, 17.02.2020 11:37




Mathematics, 17.02.2020 11:41


English, 17.02.2020 11:41

Physics, 17.02.2020 11:42

History, 17.02.2020 11:44



Mathematics, 17.02.2020 11:53





English, 17.02.2020 11:58

Health, 17.02.2020 11:59


Social Studies, 17.02.2020 12:03



