
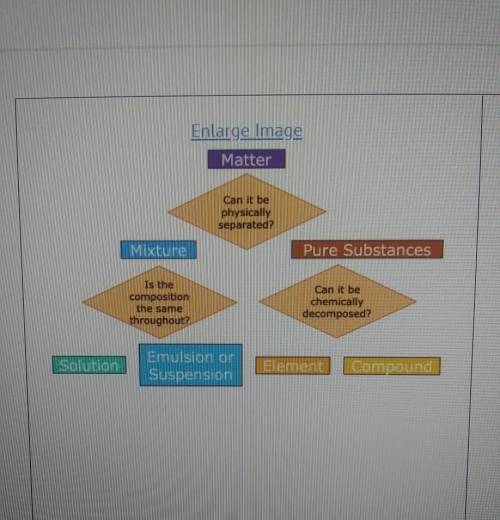
A student has a sample of ocean water that they take to science class. what steps could be taken to determine to classify the ocean water as a suspension, solution, element, or compound?
A) Examine the pH of the sample. If the pH is exactly neutral, it is a pure substance that is a compound.
B) Evaporate the water. If salt is left behind, the sample is a mixture that can be defined as a solution.
C) Let the sample settle. If the salt settles to the bottom, it is a mixture that is classified as a solution.
D) Observe the diffraction of light as it moved through the sample. If light does not scatter, it is a pure substance that is elemental.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
A student has a sample of ocean water that they take to science class. what steps could be taken to...
Questions



Mathematics, 10.04.2021 01:40




Mathematics, 10.04.2021 01:40


Mathematics, 10.04.2021 01:40





Mathematics, 10.04.2021 01:40

English, 10.04.2021 01:40

Chemistry, 10.04.2021 01:40

Physics, 10.04.2021 01:40


World Languages, 10.04.2021 01:40

Mathematics, 10.04.2021 01:40



