Chemistry, 22.11.2021 23:50 sadeed00974
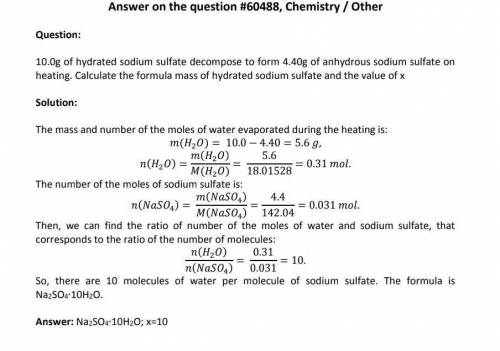
10.00g of hydrated sodium sulfate decomposes to form 4.40g Na(2)SO(4).xH(2)O -> NaSO(4) + xH(2)O of anhydrous sodium sulfate on heating. What’s the formula mass of hydrated sodium sulfate and the value of x? please help i have no clue!

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
10.00g of hydrated sodium sulfate decomposes to form 4.40g Na(2)SO(4).xH(2)O -> NaSO(4) + xH(2)O...
Questions

Computers and Technology, 05.02.2021 21:40


Arts, 05.02.2021 21:40









Mathematics, 05.02.2021 21:40

Mathematics, 05.02.2021 21:40


Mathematics, 05.02.2021 21:40


History, 05.02.2021 21:40

Mathematics, 05.02.2021 21:40