
Chemistry, 25.11.2021 14:00 spotty2093
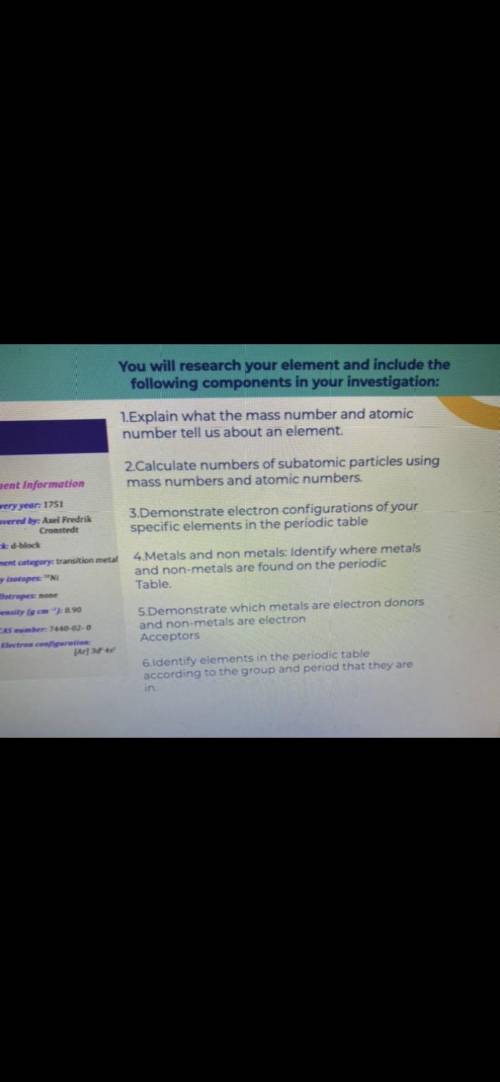
1=Explain what the mass number and atomic number tell us about an element . 2=Calculate numbers of subatomic particles using mass numbers and atomic numbers . 3=Demonstrate electron configurations of your specific elements in the periodic table 4=Metals and non metals : Identify where metals and non - metals are found on the periodic Table . 5=Demonstrate which metals are electron donors and non - metals are electron Acceptors 6=Identify elements in the periodic table according to the group and period that they are

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1


Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3

Chemistry, 23.06.2019 08:00
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2
You know the right answer?
1=Explain what the mass number and atomic number tell us about an element . 2=Calculate numbers of s...
Questions


Spanish, 25.01.2021 20:50

Spanish, 25.01.2021 20:50


English, 25.01.2021 20:50




Mathematics, 25.01.2021 20:50


Mathematics, 25.01.2021 20:50











