Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
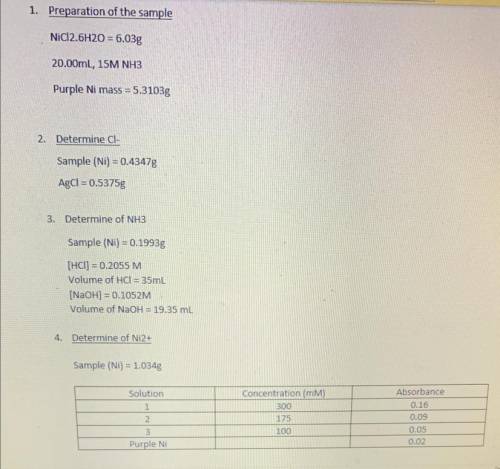
Using your three values for percent, calculate the empirical formula for the compound. Write the bal...
Questions



English, 11.11.2021 01:00

Social Studies, 11.11.2021 01:00



Mathematics, 11.11.2021 01:00

Arts, 11.11.2021 01:00


Mathematics, 11.11.2021 01:00


Chemistry, 11.11.2021 01:00

Mathematics, 11.11.2021 01:00

History, 11.11.2021 01:00


History, 11.11.2021 01:00

Mathematics, 11.11.2021 01:00

SAT, 11.11.2021 01:00

Mathematics, 11.11.2021 01:00