
Chemistry, 30.11.2021 03:40 ralucacoriciuc2482
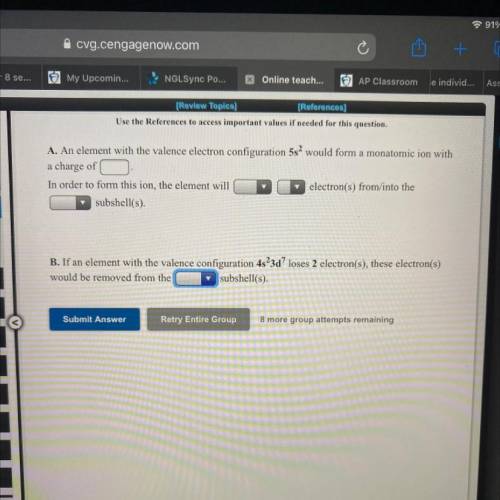
A. An element with the valence electron configuration 5s2 would form a monatomic ion with
a charge of _.
In order to form this ion, the element will_(lose, gain) _(1-8) electron(s) from/into the ___(s, p,d, f,s+p, s+d, p+d) subshell(s).
B. If an element with the valence configuration 4s2 3d7 loses 2 electron(s), these electron(s)
would be removed from the ___(s, p,d, f,s+p, s+d, p+d)
subshell(s).

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3
You know the right answer?
A. An element with the valence electron configuration 5s2 would form a monatomic ion with
a charge...
Questions


Mathematics, 24.02.2021 04:00




History, 24.02.2021 04:00



Mathematics, 24.02.2021 04:00




Computers and Technology, 24.02.2021 04:00

History, 24.02.2021 04:00


Mathematics, 24.02.2021 04:00

Chemistry, 24.02.2021 04:00

English, 24.02.2021 04:00


Mathematics, 24.02.2021 04:00



