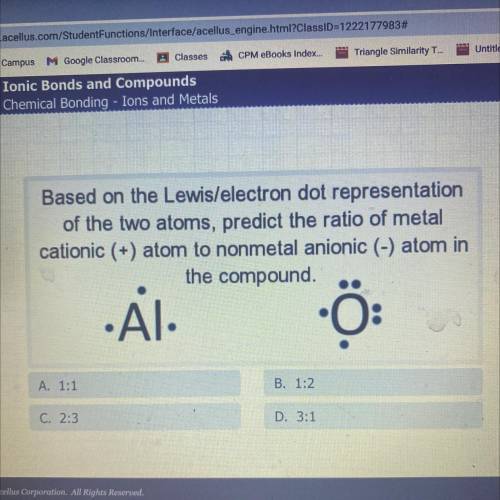
Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
...

Chemistry, 30.11.2021 22:40 caseychandler030418
Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
cationic (+) atom to nonmetal anionic (-) atom in
the compound.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Read the given expression. x = number of protons − number of core electrons which of the following explains the identity of x and its trends across a period? x is the effective nuclear charge, and it remains constant across a period. x is the screening constant, and it remains constant across a period. x is the effective nuclear charge, and it increases across a period. x is the screening constant, and it increases across a period.
Answers: 1



You know the right answer?
Questions

Mathematics, 20.10.2019 18:30

Biology, 20.10.2019 18:30



Mathematics, 20.10.2019 18:30

Mathematics, 20.10.2019 18:30

History, 20.10.2019 18:30


Mathematics, 20.10.2019 18:30



Mathematics, 20.10.2019 18:30

English, 20.10.2019 18:30

Mathematics, 20.10.2019 18:30




English, 20.10.2019 18:30


English, 20.10.2019 18:30



