The Mole
7
Acellus
Now that you know the scaling factor, simply
multiply each su...

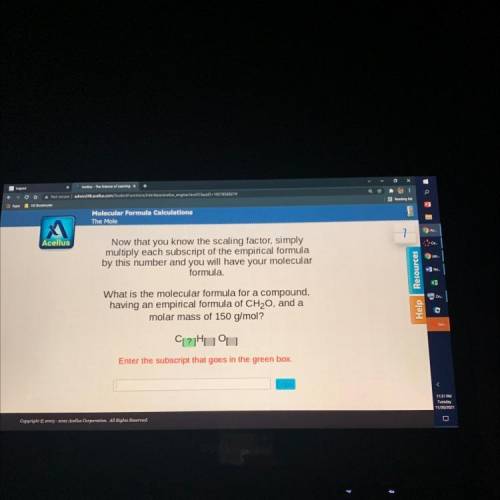
The Mole
7
Acellus
Now that you know the scaling factor, simply
multiply each subscript of the empirical formula
by this number and you will have your molecular
formula.
on Resources
What is the molecular formula for a compound,
having an empirical formula of CH20, and a
molar mass of 150 g/mol?
CL?Huu
Enter the subscript that goes in the green box.
Enter

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
Questions



Biology, 12.02.2020 00:35




Physics, 12.02.2020 00:35

History, 12.02.2020 00:35




Mathematics, 12.02.2020 00:35


Mathematics, 12.02.2020 00:35


Mathematics, 12.02.2020 00:35

Mathematics, 12.02.2020 00:35

Physics, 12.02.2020 00:35

Business, 12.02.2020 00:35



