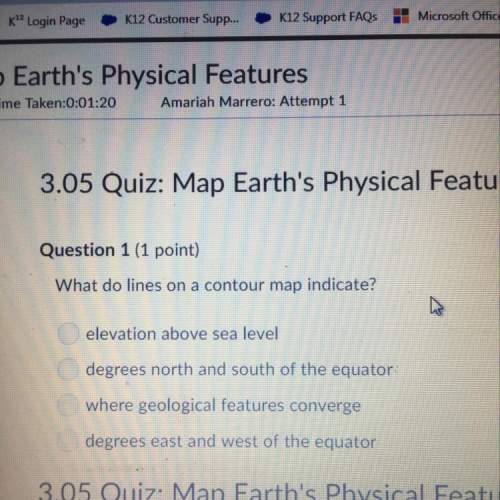
A 139.9 g piece of copper (specific heat 0.380 J/g・°C) is heated and then placed into 400.0 g of water initially at 20.7°C. The water increases in temperature to 22.2°C. What is the initial temperature (in °C) of the copper? (The specific heat of water is 4.184 J/g・°C).

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1
You know the right answer?
A 139.9 g piece of copper (specific heat 0.380 J/g・°C) is heated and then placed into 400.0 g of wat...
Questions

Mathematics, 28.06.2019 10:00


Mathematics, 28.06.2019 10:00

Social Studies, 28.06.2019 10:00

Social Studies, 28.06.2019 10:00



Spanish, 28.06.2019 10:00


Business, 28.06.2019 10:00

Mathematics, 28.06.2019 10:00

Chemistry, 28.06.2019 10:00

French, 28.06.2019 10:00


Mathematics, 28.06.2019 10:00

Health, 28.06.2019 10:00

Mathematics, 28.06.2019 10:00


English, 28.06.2019 10:00

Mathematics, 28.06.2019 10:00