
Chemistry, 02.12.2021 02:30 kiahbryant12
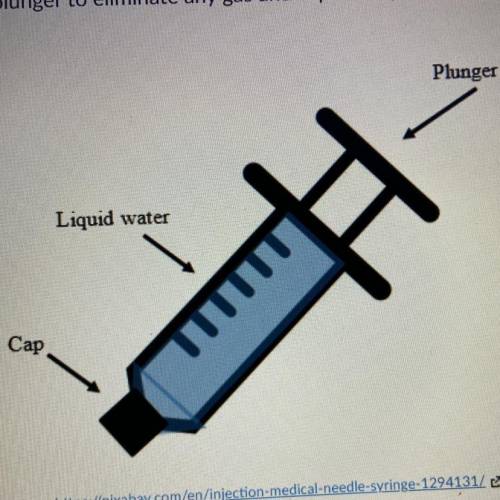
A student is convinced that he is strong enough to compress a liquid. He draws 5 mL of water into a syringe. He pushes the plunger to illuminate any gas in caps the syringe. His set up looks like the image below. With the cap fixed on he pushes the plunger into the syringe with as much force as he can. Which of the following do you expect to happen? 1. He will be able to compress than a liquid because the molecules of water are attracted to one another. 2. He will not be able to compress the liquid because he is not strong enough to break the water bonds. 3. He will not be able to compress the liquid Because the molecules of a liquid to close to because the molecules of a liquid too close together. 4. He will be able to compress than a liquid because there is a lot of space in between the molecules.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

You know the right answer?
A student is convinced that he is strong enough to compress a liquid. He draws 5 mL of water into a...
Questions


Mathematics, 18.03.2021 18:20

Mathematics, 18.03.2021 18:20

Mathematics, 18.03.2021 18:20



Mathematics, 18.03.2021 18:20

Mathematics, 18.03.2021 18:20

Computers and Technology, 18.03.2021 18:20



Mathematics, 18.03.2021 18:20



Chemistry, 18.03.2021 18:20

English, 18.03.2021 18:20

Mathematics, 18.03.2021 18:20


History, 18.03.2021 18:20

Social Studies, 18.03.2021 18:20



