9.
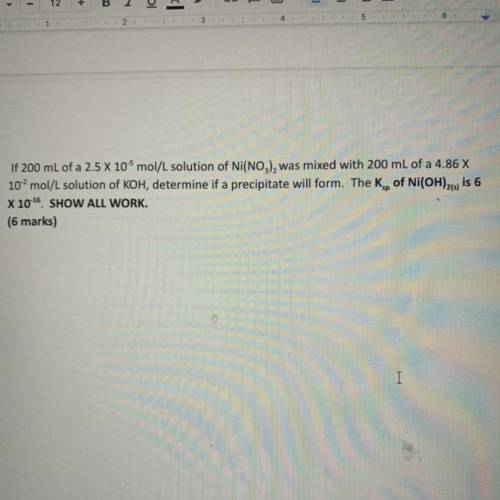
If 200 mL of a 2.5 x 10-5 mol/L solution of Ni(NO3), was mixed with 200 mL of a 4.86 X
10...

Chemistry, 02.12.2021 18:10 kstyleszdance
9.
If 200 mL of a 2.5 x 10-5 mol/L solution of Ni(NO3), was mixed with 200 mL of a 4.86 X
102 mol/L solution of KOH, determine if a precipitate will form. The Kg, of Ni(OH)21, is 6
X 10-16 SHOW ALL WORK.
(6 marks)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
You know the right answer?
Questions





Mathematics, 17.10.2019 16:30



Mathematics, 17.10.2019 16:30





Mathematics, 17.10.2019 16:30




Mathematics, 17.10.2019 16:30

History, 17.10.2019 16:30





