
Chemistry, 02.12.2021 21:30 maciemarklin3032
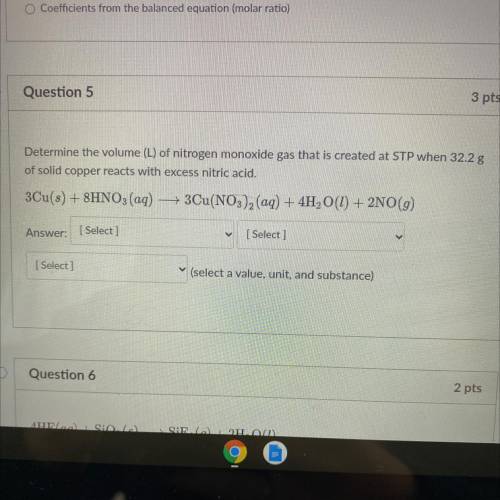
Determine the volume (L) of nitrogen monoxide gas that is created at STP when 32.2 g
of solid copper reacts with excess nitric acid.
3Cu(s) + 8HNO3(aq) — 3Cu(NO3)2 (aq) + 4H2O(1) + 2NO(g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
You know the right answer?
Determine the volume (L) of nitrogen monoxide gas that is created at STP when 32.2 g
of solid copp...
Questions


History, 04.12.2021 04:40

Business, 04.12.2021 04:40








Mathematics, 04.12.2021 04:40


English, 04.12.2021 04:40



Mathematics, 04.12.2021 04:40


Social Studies, 04.12.2021 04:40


Health, 04.12.2021 04:40



