
Chemistry, 02.12.2021 21:30 Hollywood0122
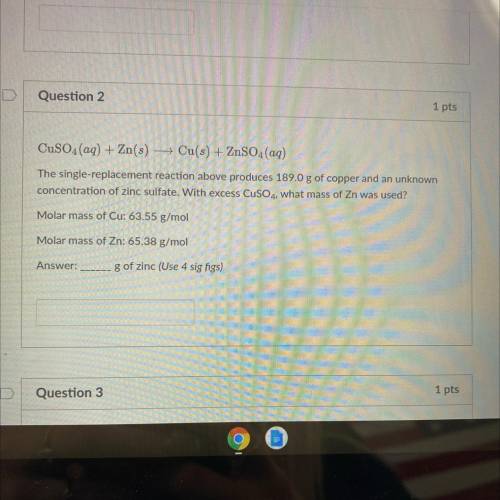
CuSO4(aq) + Zn(s) + Cu(s) + ZnSO4(aq)
The single-replacement reaction above produces 189.0 g of copper and an unknown
concentration of zinc sulfate. With excess CuSO4, what mass of Zn was used?
Molar mass of Cu: 63.55 g/mol
Molar mass of Zn: 65.38 g/mol
g of zinc (Use 4 sig figs)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

You know the right answer?
CuSO4(aq) + Zn(s) + Cu(s) + ZnSO4(aq)
The single-replacement reaction above produces 189.0 g of co...
Questions










Mathematics, 29.01.2021 21:30

Mathematics, 29.01.2021 21:30








Mathematics, 29.01.2021 21:30

Arts, 29.01.2021 21:30



