
Chemistry, 03.12.2021 01:50 janelisse199820
The following cations and anions in solution are mixed together, one pair at a time Hg+, K+, Al3+ and I-, S2-, CO3 2- Write a net ionic equation for each precipitate that forms, including states
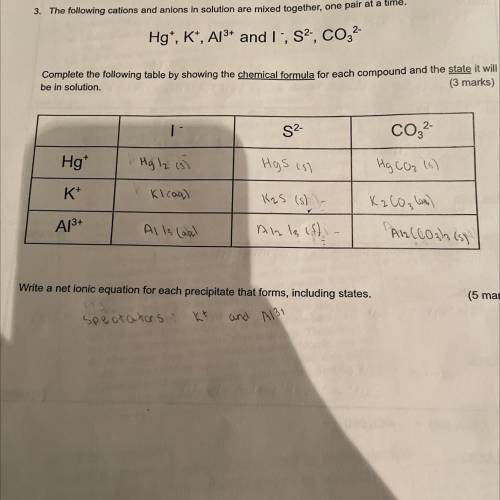

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3


Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
The following cations and anions in solution are mixed together, one pair at a time Hg+, K+, Al3+ an...
Questions



Biology, 08.06.2021 18:20

Mathematics, 08.06.2021 18:20

Mathematics, 08.06.2021 18:20




Mathematics, 08.06.2021 18:20








Mathematics, 08.06.2021 18:20


Mathematics, 08.06.2021 18:20



