
Chemistry, 04.12.2021 01:30 44chandracloutier
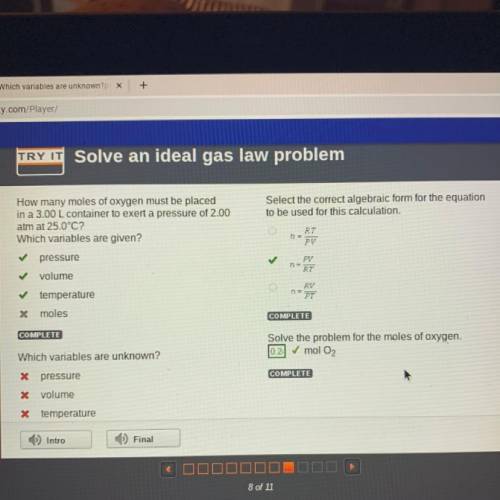
How many moles of oxygen must be placed
in a 3.00 L container to exert a pressure of 2.00
atm at 25.0°C?
Which variables are given?
Select the correct algebraic form for the equation
to be used for this calculation.
RT
PV
pressure
✓
PV
RT
✓
volume
22
RV
PT
temperature
* moles
COMPLETE
COMPLETE
Solve the problem for the moles of oxygen.
0.2 mol O2
Which variables are unknown?
X pressure
COMPLETE
X volume
X temperature

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
How many moles of oxygen must be placed
in a 3.00 L container to exert a pressure of 2.00
at...
at...
Questions


Mathematics, 29.04.2021 23:20





Mathematics, 29.04.2021 23:20

English, 29.04.2021 23:20







Mathematics, 29.04.2021 23:20

Chemistry, 29.04.2021 23:20

Mathematics, 29.04.2021 23:20

Biology, 29.04.2021 23:20

Mathematics, 29.04.2021 23:20

Mathematics, 29.04.2021 23:20



