Chemistry, 05.12.2021 14:00 idontknow1993
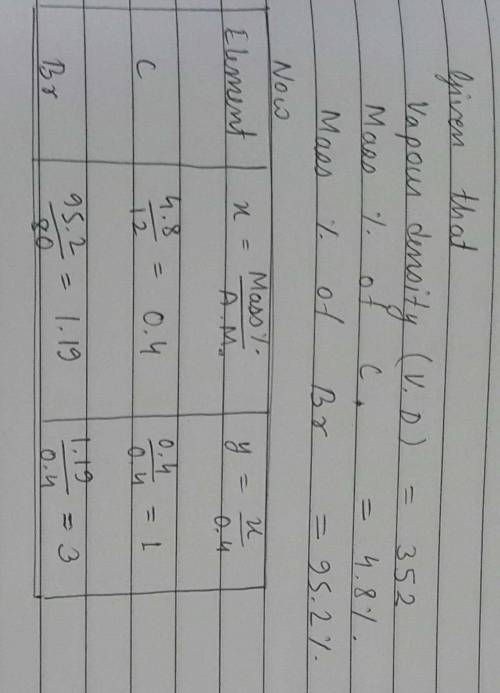
2. A compound with an empirical formula of C2H3Br2 has a molar mass of 373.69 g/mol. What is
the molecular formula?
(The molar mass of C2H3Br2 = 186.85 g/mol)
E) C4H6Br4
А) C2H3Br2
B) CHBr
C) C6H9Br6 D) C4H6Br2
(0.5 Points)
A

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
2. A compound with an empirical formula of C2H3Br2 has a molar mass of 373.69 g/mol. What is
the m...
Questions

Mathematics, 10.09.2021 03:00




Mathematics, 10.09.2021 03:00

Biology, 10.09.2021 03:00

Mathematics, 10.09.2021 03:00

Mathematics, 10.09.2021 03:00

Chemistry, 10.09.2021 03:00



Mathematics, 10.09.2021 03:00

Mathematics, 10.09.2021 03:00

Social Studies, 10.09.2021 03:00

Mathematics, 10.09.2021 03:00


Business, 10.09.2021 03:00



History, 10.09.2021 03:00