
Chemistry, 05.12.2021 22:30 emilypzamora11
Q1. How many moles of air are in a 2.8 x 106 L balloon at 20 °C and 750 mmHg of pressure? If the average molar mass of air is 28 g/mol, how many tons of air is this? (1 ton = 2,000 lb)?
Q2. This same balloon is heated from 20 °C to 100 °C keeping the volume and pressure constant. Calculate the new number of tons of gas inside the balloon.
Q3. Calculate the density of the air in the hot air balloon when the air inside is 100 °C in kg/m3.
(Hint: Use the mass you already calculated in question 2!)
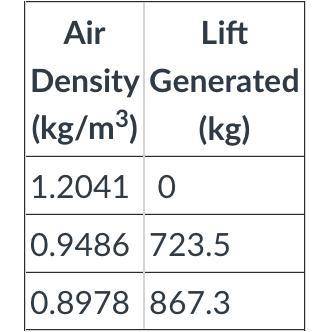
Q4. Use the table attached to determine if the balloon will float at this temperature. (Note: the total mass of the balloon and basket is 723.5 kg. You will need at least that much lift.)

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
Q1. How many moles of air are in a 2.8 x 106 L balloon at 20 °C and 750 mmHg of pressure? If the ave...
Questions

Mathematics, 05.12.2020 01:00


Mathematics, 05.12.2020 01:00

Law, 05.12.2020 01:00

Business, 05.12.2020 01:00

Mathematics, 05.12.2020 01:00




Health, 05.12.2020 01:00


Mathematics, 05.12.2020 01:00

History, 05.12.2020 01:00

Mathematics, 05.12.2020 01:00

Mathematics, 05.12.2020 01:00

Mathematics, 05.12.2020 01:00




Geography, 05.12.2020 01:00



