
Chemistry, 06.12.2021 03:40 loganparrish2488
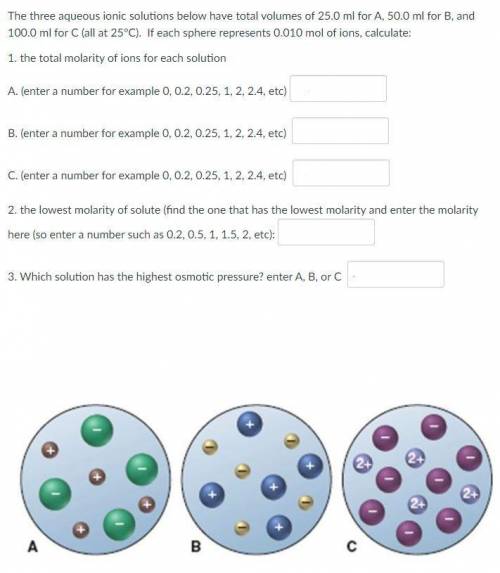
The three aqueous ionic solutions below have total volumes of 25.0 ml for A, 50.0 ml for B, and 100.0 ml for C (all at 25°C). If each sphere represents 0.010 mol of ions, calculate:
1. the total molarity of ions for each solution
2. the lowest molarity of solute
3. Which solution has the highest osmotic pressure?
See the picture attached.
My answers:
1.
A. 3.2
B. 2
C. 1.2
2. 0.4
3. A
Am I right?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1
You know the right answer?
The three aqueous ionic solutions below have total volumes of 25.0 ml for A, 50.0 ml for B, and 100....
Questions

Spanish, 03.01.2020 06:31




Social Studies, 03.01.2020 06:31


Biology, 03.01.2020 06:31


Chemistry, 03.01.2020 06:31



Biology, 03.01.2020 06:31

Mathematics, 03.01.2020 06:31

Geography, 03.01.2020 06:31


Mathematics, 03.01.2020 06:31




Mathematics, 03.01.2020 06:31



