Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

You know the right answer?
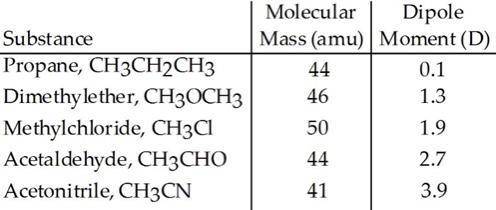
Based on molecular mass and dipole moment of the five compounds in the table below, which should hav...
Questions


English, 31.03.2021 22:00

Mathematics, 31.03.2021 22:00


Geography, 31.03.2021 22:00

Arts, 31.03.2021 22:00

English, 31.03.2021 22:00

English, 31.03.2021 22:00

Mathematics, 31.03.2021 22:00

Mathematics, 31.03.2021 22:00


Mathematics, 31.03.2021 22:00

Biology, 31.03.2021 22:00

Mathematics, 31.03.2021 22:00

Health, 31.03.2021 22:00



Mathematics, 31.03.2021 22:00

World Languages, 31.03.2021 22:00

Physics, 31.03.2021 22:00