
Chemistry, 07.12.2021 19:40 isaiahcannon5709
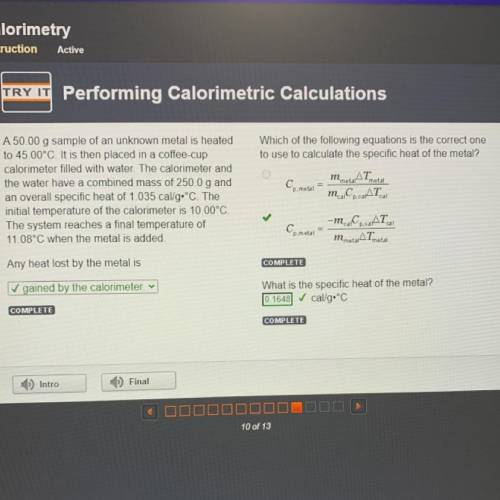
Which of the following equations is the correct one
to use to calculate the specific heat of the metal?
AT
Cp. metal
m. Co. AT
metal
A 50.00 g sample of an unknown metal is heated
to 45.00°C. It is then placed in a coffee-cup
calorimeter filled with water. The calorimeter and
the water have a combined mass of 250.0 g and
an overall specific heat of 1.035 cal/g•°C. The
initial temperature of the calorimeter is 10.00°C.
The system reaches a final temperature of
11.08°C when the metal is added.
metal
cal
Ce metal
-m. C.CAT
m metarATmetal
COMPLETE
Any heat lost by the metal is
✓ gained by the calorimeter v
What is the specific heat of the metal?
cal/g.°C
COMPLETE
DONE

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Supongamos que estás estudiando dos estrellas. ambas estrellas tienen la misma magnitud aparente, pero la estrella a tiene una magnitud absoluta mayor que la estrella b. ¿que puedes decir acerca de la distancia a la tierra de estas dos estrellas?
Answers: 3


Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
Which of the following equations is the correct one
to use to calculate the specific heat of the m...
Questions


Biology, 18.10.2020 01:01


Health, 18.10.2020 01:01


Mathematics, 18.10.2020 01:01

Chemistry, 18.10.2020 01:01


English, 18.10.2020 01:01



Advanced Placement (AP), 18.10.2020 01:01

English, 18.10.2020 01:01


Mathematics, 18.10.2020 01:01



Arts, 18.10.2020 01:01




