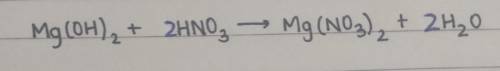Chemistry, 07.12.2021 23:20 Alexisgrab
When magnesium hydroxide reacts with nitric acid, it produces magnesium nitrate and water. Balance the following reaction:
__ Mg(OH)2 + __ HNO3 → __ Mg(NO3)2 + __ H2O
Please answer correctly. I will report you If you steal my points. I will also mark whoever answers first the brainliest (unless my computer doesn't let me)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
When magnesium hydroxide reacts with nitric acid, it produces magnesium nitrate and water. Balance t...
Questions

Mathematics, 17.10.2019 23:30

English, 17.10.2019 23:30


Arts, 17.10.2019 23:30


Biology, 17.10.2019 23:30

Mathematics, 17.10.2019 23:30


World Languages, 17.10.2019 23:30

Biology, 17.10.2019 23:30

Social Studies, 17.10.2019 23:30


Mathematics, 17.10.2019 23:30


English, 17.10.2019 23:30


Social Studies, 17.10.2019 23:30


History, 17.10.2019 23:30

Social Studies, 17.10.2019 23:30