
Chemistry, 08.12.2021 03:10 ballin3294
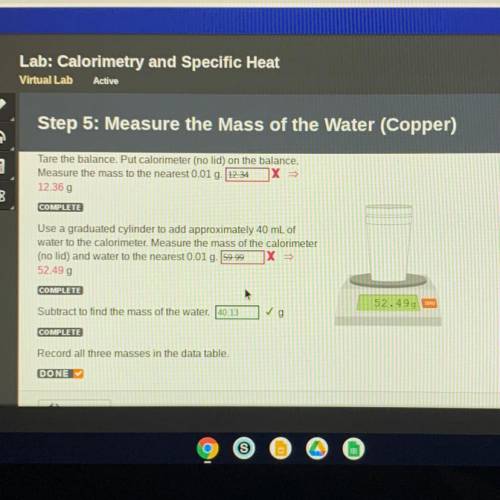
Step 5: Measure the Mass of the Water (Copper)
Tare the balance. Put calorimeter (no lid) on the balance.
Measure the mass to the nearest 0.01 g. 12.34 1X >>
12.36 g
COMPLETE
Use a graduated cylinder to add approximately 40 mL of
water to the calorimeter. Measure the mass of the calorimeter
(no lid) and water to the nearest 0.01 g. 59.99
X =
52.49 g
COMPLETE
52.499 250
Subtract to find the mass of the water. 40.13
✓g
COMPLETE
Record all three masses in the data table.
DONE

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1

Chemistry, 24.06.2019 01:30
What is the balanced equation of this chemical reaction ?
Answers: 3
You know the right answer?
Step 5: Measure the Mass of the Water (Copper)
Tare the balance. Put calorimeter (no lid) on the b...
Questions












Computers and Technology, 28.05.2021 04:30

Computers and Technology, 28.05.2021 04:30

Physics, 28.05.2021 04:30

Mathematics, 28.05.2021 04:30







