Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

You know the right answer?
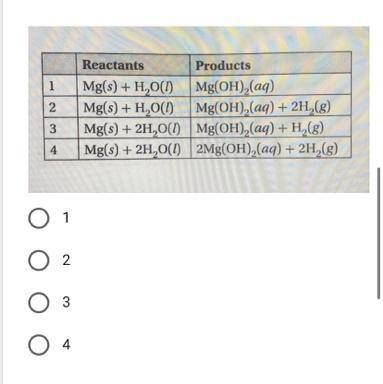
Analyze the reaction of solid magnesium and water. Which pair of reactants and products in the table...
Questions

Mathematics, 23.06.2021 06:20

Mathematics, 23.06.2021 06:20

Mathematics, 23.06.2021 06:20

Biology, 23.06.2021 06:20

Mathematics, 23.06.2021 06:20

Mathematics, 23.06.2021 06:20

Mathematics, 23.06.2021 06:20


English, 23.06.2021 06:20


Social Studies, 23.06.2021 06:20



Mathematics, 23.06.2021 06:20




Mathematics, 23.06.2021 06:20